**TIM-3 Therapy for Alzheimer’s** represents a groundbreaking advance in the quest for effective Alzheimer’s treatment, harnessing the body’s immune system to enhance brain health. Recent studies show that inhibiting TIM-3, a checkpoint molecule, can invigorate microglia function—the brain’s resident immune cells—allowing them to clear harmful amyloid plaque that contributes to cognitive decline. This approach not only aims to improve memory and cognitive performance in Alzheimer’s patients but also aligns with the established principles of cancer immunotherapy. By repurposing strategies effective against cancer, researchers hope to offer new hope in the fight against Alzheimer’s disease. As we delve deeper into the mechanisms at play, the potential for cognitive improvement through TIM-3 therapy becomes increasingly promising, suggesting a new frontier in addressing immune system dysfunction in Alzheimer’s.
When exploring innovative treatments for Alzheimer’s, the concept of **TIM-3 Therapy** emerges as a vital focal point. This emerging strategy revolves around the modulation of immune checkpoint molecules, which have traditionally been associated with cancer therapies, but are now being repurposed to address Alzheimer’s pathology. By enhancing the role of microglial cells—key players in brain immunity—this therapy offers a pathway to clear amyloid plaques that hinder cognitive function. The strategy reflects a shift towards understanding how immune responses can be recalibrated to foster cognitive resilience in aging populations. As research progresses, the hope is that this novel approach will pave the way for breakthrough advancements in Alzheimer’s treatment.
Understanding TIM-3 and Alzheimer’s Disease
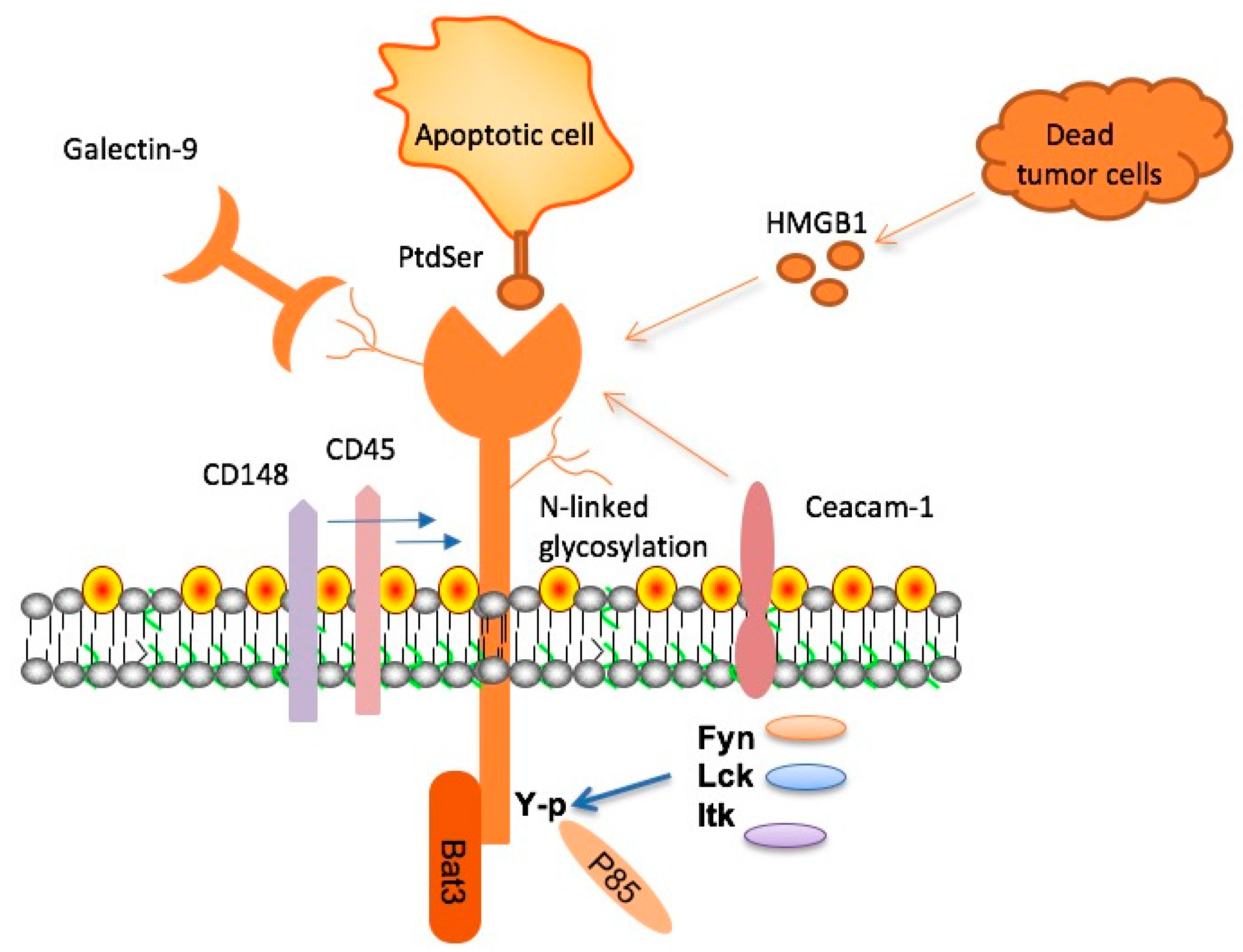
TIM-3, or T-cell immunoglobulin and mucin-domain containing-3, is a checkpoint molecule that plays a critical role in the immune response. In the context of Alzheimer’s disease, elevated TIM-3 levels on microglia—blood-brain barrier-resident immune cells—prevent them from clearing amyloid-beta plaques from the brain. This plaque accumulation is a hallmark of Alzheimer’s, leading to neuroinflammation and cognitive decline. Recent studies suggest that targeting TIM-3 could enhance microglia function and promote the clearance of these harmful plaques.
Research indicates that nearly 90% of Alzheimer’s cases are late-onset, closely associated with genetic factors such as polymorphisms in the TIM-3 gene, HAVCR2. The discovery of this connection not only sheds light on the pathophysiology of the disease but also opens new avenues for therapeutic interventions. By understanding how TIM-3 interacts with immune regulatory pathways in the context of Alzheimer’s, researchers can develop strategies that leverage this knowledge for cognitive improvement in affected individuals.
The Role of the Immune System in Alzheimer’s
The immune system’s interplay with Alzheimer’s disease is complex, particularly in how microglia respond to amyloid-beta plaques. Microglia are the brain’s resident immune cells responsible for maintaining homeostasis by clearing dead cells and debris. However, in Alzheimer’s patients, these innate immune responses become dysregulated, mainly due to inhibitory signals from checkpoint molecules like TIM-3. This dysregulation leads to reduced microglial activation and an inability to effectively clear plaques, underlining the importance of immune system functionality in Alzheimer’s treatment.
During the progression of Alzheimer’s, microglia that should be functioning as defenders become dysfunctional. They fail to adequately respond to the plaque buildup as their TIM-3 expression inhibits their phagocytic actions. This dynamic points to potential therapeutic strategies that could restore normal microglial function through TIM-3 modulation, which might not only help in clearing plaques but also reduce neuroinflammation, thus potentially slowing cognitive decline.
Potential of TIM-3 Therapy in Alzheimer’s Treatment
The potential of TIM-3 therapy for Alzheimer’s patients is particularly promising. By applying anti-TIM-3 antibodies or small molecules to inhibit TIM-3’s function, researchers aim to revive microglial activity, allowing these cells to target and remove amyloid-beta plaques effectively. Initial studies in mouse models have shown cognitive improvement following TIM-3 inhibition, indicating that this approach might translate into meaningful therapeutic interventions for the human population suffering from Alzheimer’s.
Importantly, the strategy of repurposing existing cancer immunotherapy treatments, which target TIM-3 for tumor suppression, highlights a revolutionary cross-disciplinary approach. By leveraging the knowledge gained from cancer research, it is possible to address Alzheimer’s disease with an immune-modulatory strategy that has previously demonstrated efficacy in other contexts. This paradigm shift not only expands the horizons of Alzheimer’s treatment but also underscores the critical role played by the immune system in neurodegenerative diseases.
Microglia Function and Cognitive Improvement
Microglia, as the brain’s primary immune cells, have a dual role in both the maintenance of neuronal health and the response to neurological diseases. Their ability to modulate synapses during development and prune unused connections showcases their essential function in learning and memory. However, with Alzheimer’s disease, timely microglial intervention is necessary to clear the accumulating debris and plaques that hinder cognitive functionality. Therapeutic strategies aimed at enhancing microglial function could thus hold the key to restoring cognitive abilities in affected patients.
An exciting area of research is investigating how targeting the TIM-3 signaling pathway could rejuvenate microglial function, thus leading to cognitive improvement. By freeing microglia from the inhibitory effects of TIM-3, there’s potential not only to enhance plaque removal but also to restore synaptic integrity. Improved synaptic function could translate to better memory retention and navigational abilities in patients, addressing a fundamental challenge posed by Alzheimer’s disease.
Challenges in Alzheimer’s Therapies: The Role of TIM-3
Despite recent advancements in Alzheimer’s therapies, many clinical trials have reported only minor cognitive improvements. Existing anti-amyloid therapies often face challenges such as low brain permeability and vascular safety issues, leading to adverse effects. However, the selective expression of TIM-3 on microglia presents a unique opportunity to devise targeted treatments that enhance microglial activity without engaging broader immune responses, thereby potentially reducing the risk of side effects.
The challenge remains to validate TIM-3 therapy’s effectiveness in human clinical trials, as animal studies, although promising, may not fully mimic human biology. Continued research focusing on the role of TIM-3 and its impact on microglial function is crucial. Researchers are hopeful that with the right interventions, not only can plaque accumulation be tackled, but also patient cognition can be meaningfully preserved and improved.
The Future of Alzheimer’s Treatment with Immunotherapy
As researchers continue to unveil the complex mechanisms of Alzheimer’s disease, immunotherapy emerges as a beacon of hope. With the success seen in cancer treatments that involve checkpoint inhibition such as TIM-3, researchers are exploring similar pathways in neurodegeneration. The use of immunotherapeutics may redefine approaches to cognitive decline associated with Alzheimer’s, allowing for the systemic rejuvenation of the brain’s immune responses.
Future therapies could integrate TIM-3 modulation with existing Alzheimer’s strategies, fostering a multifaceted approach that combines cognitive therapies with immune system enhancements. In doing so, the aim would be to not only address the pathological features of Alzheimer’s but also restore overall cognitive capacity and quality of life for patients.
Exploring Gene Therapy Approaches for Alzheimer’s
Gene therapy presents a groundbreaking avenue for treating Alzheimer’s disease by targeting genetic factors involved in the disease progression. With the identification of the TIM-3 gene as a risk factor for late-onset Alzheimer’s, researchers are investigating the feasibility of directly modifying these genes. Targeted gene therapy could provide a permanent solution by essentially pre-treating the brain to prevent plaque accumulation before symptoms begin.
By utilizing CRISPR technology or similar gene-editing techniques, it may be possible to alter the expression of harmful genes or enhance protective mechanisms within the brain. This innovative approach not only holds promise for addressing Alzheimer’s but also complements existing therapies focused on the immune response, creating a comprehensive plan for patients facing this debilitating condition.
Cognitive Behavior and Alzheimer’s Disease
Understanding the cognitive behavior of Alzheimer’s patients is essential for developing effective treatments. Patients often exhibit memory loss, confusion, and difficulties in navigating familiar environments, profoundly impacting their daily lives. Recent studies have shown that interventions targeting microglial function—like TIM-3 inhibition—can significantly improve memory-related tasks in animal models, underscoring the connection between brain health and cognitive behaviors.
By examining how cognitive behavior manifests in individuals with Alzheimer’s, particularly in their ability to remember and navigate spaces, we can tailor therapies that specifically address these deficits. Insights into the behavioral patterns of Alzheimer’s patients could also inform care strategies, enabling a more holistic approach to treatment that encompasses not only biological interventions but also cognitive rehabilitation methods.
The Importance of Collaboration in Alzheimer’s Research
Advancements in Alzheimer’s research rely heavily on collaborative efforts among scientists, clinicians, and institutions. The complexity of this neurodegenerative disease requires a multidisciplinary approach that includes immunology, neurology, and genetic research. By pooling resources and insights, researchers can collectively address the challenges posed by Alzheimer’s and work towards innovative therapies, such as those involving TIM-3.
Collaboration among institutions, like the partnerships observed between various Harvard Medical School departments, significantly enhances the research productivity and accessibility to advanced technologies. These synergies are critical for accelerating discoveries that could lead to effective treatments for Alzheimer’s disease, propelling the field forward toward meaningful patient outcomes.
Frequently Asked Questions
What is TIM-3 therapy for Alzheimer’s and how does it work?
TIM-3 therapy for Alzheimer’s focuses on blocking the TIM-3 checkpoint molecule, which inhibits microglia from clearing amyloid plaques in the brain. By turning off TIM-3, the microglial cells can effectively attack and remove these harmful plaques, potentially leading to cognitive improvement in Alzheimer’s patients.
Can TIM-3 therapy improve cognitive function in Alzheimer’s patients?
Emerging research suggests that TIM-3 therapy can enhance cognitive function by restoring microglia’s ability to clear amyloid plaques. Studies conducted on mice have shown that deleting the TIM-3 gene improved their memory and navigational skills, indicating a promising pathway for Alzheimer’s treatment.
What role does microglia play in Alzheimer’s disease and how is it affected by TIM-3?
Microglia act as the brain’s immune cells and are responsible for clearing amyloid beta plaques associated with Alzheimer’s. In Alzheimer’s patients, the expression of the TIM-3 molecule prevents microglia from effectively removing these plaques, leading to cognitive decline. TIM-3 therapy aims to free microglia to perform this crucial function.
How does TIM-3 therapy compare to traditional Alzheimer’s treatments?
Unlike traditional Alzheimer’s treatments that primarily target amyloid plaques directly, TIM-3 therapy seeks to enhance the immune response by allowing microglia to resume their role in plaque clearance. This novel approach may address underlying mechanisms rather than just symptoms, potentially leading to more significant cognitive improvements.
What are the potential side effects of TIM-3 therapy for Alzheimer’s?
Since TIM-3 therapy involves modifying immune cell functions, possible side effects could include an increased risk of autoimmune reactions or inflammation. However, research is ongoing to ensure that TIM-3 therapy is safe and effective before clinical applications in Alzheimer’s treatment.
Is TIM-3 therapy already available for Alzheimer’s patients?
Currently, TIM-3 therapy is still in the research phase, with studies focusing on its efficacy in animal models. Human clinical trials are being planned to assess the treatment’s safety and effectiveness in Alzheimer’s patients, but it is not yet available as a standard treatment option.
What are the implications of TIM-3 research for future Alzheimer’s treatments?
Research on TIM-3 has significant implications for future Alzheimer’s therapies. If successful, TIM-3-based treatments could provide a new avenue for intervention, addressing both plaque clearance and immune regulation in the brain, and possibly leading to breakthroughs that improve cognitive outcomes for patients.
| Key Point | Details |
|---|---|
| Research Publication | Published in Nature, highlighting new therapy possibilities for Alzheimer’s using TIM-3 research. |
| Role of TIM-3 | TIM-3 is an inhibitory molecule that limits the action of microglia, the brain’s immune cells. |
| Microglia and Plaque Clearance | By eliminating TIM-3 expression, microglia are allowed to clear amyloid plaques, improving memory in mice. |
| Late-Onset Alzheimer’s | Most Alzheimer’s cases (90-95%) are late-onset, with TIM-3 linked to genetic risk. |
| Potential Treatment | TIM-3 therapy could involve anti-TIM-3 antibodies to block its function and restore cognitive abilities. |
Summary
TIM-3 Therapy for Alzheimer’s shows promising potential as a groundbreaking approach to combat this debilitating disease. This research suggests that by targeting the TIM-3 molecule, which inhibits the brain’s immune response, we can facilitate the clearance of harmful amyloid plaques and significantly improve cognitive functions in models of Alzheimer’s. As researchers move towards human trials, the hope is that a TIM-3 targeted therapy could turn the tide against Alzheimer’s, offering new avenues for treatment where previous methods have fallen short.