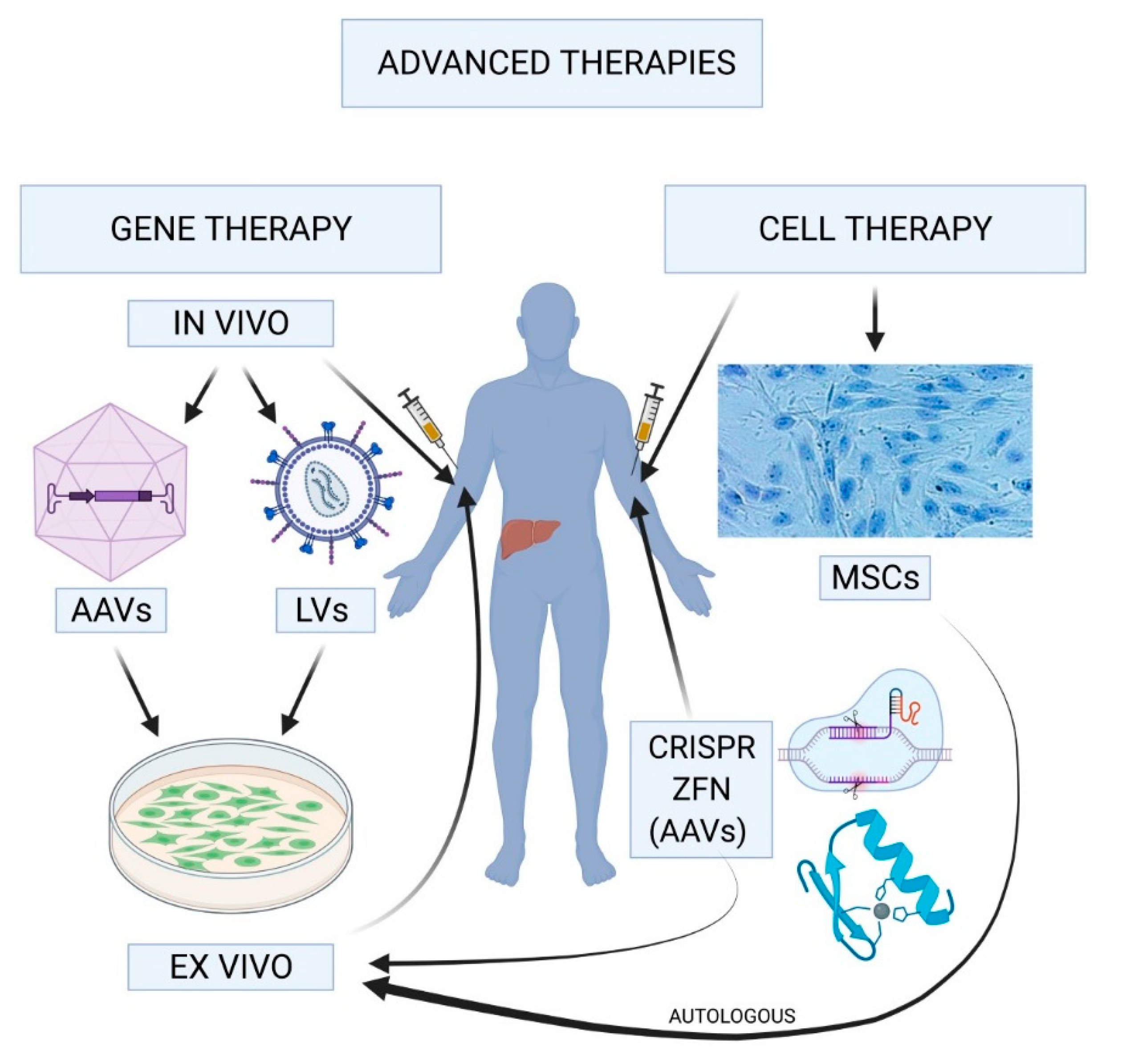
Gene therapy for hemophilia marks a groundbreaking advancement in the treatment of this challenging condition, providing hope for patients like Terence Blue, who have managed the complexities of hemophilia B for decades. Through innovative approaches such as Hemgenix gene therapy, developed by CSL Behring, patients may experience significant reductions in their reliance on traditional clotting factor IX injections. This form of hemophilia treatment seeks to correct the genetic mutations that cause the disorder, transforming the lives of those affected. With the FDA approving Hemgenix in November 2022, the medical community is witnessing a pivotal moment in gene therapy breakthroughs that could redefine how hemophilia is managed. As we delve deeper into the potential of these therapies, we recognize the hopeful strides made in addressing the challenges associated with hemophilia and the promise of a more sustainable quality of life for patients.
Hemophilia, often characterized by a deficiency in blood clotting factors, is a life-altering condition primarily affecting males due to its genetic link on the X chromosome. Revolutionary treatments, including genetic modifications designed to enhance or replace dysfunctional genes, are gaining traction as potential solutions to this lifelong challenge. Patients with hemophilia B, who have long depended on regular infusions of clotting factor IX, now have access to pioneering therapies like Hemgenix, which aim to alleviate their dependence on traditional treatment regimens. The recent approval of such therapies signifies not just a medical triumph but also a shift towards a future where gene manipulation could lead to substantial, lasting improvements in patient health. As research continues, the horizon appears promising for those seeking respite from the burdens of hemophilia.
Understanding Hemophilia and Its Challenges
Hemophilia is a genetic disorder that significantly affects the body’s ability to clot blood effectively. The most common types are hemophilia A and hemophilia B, with the latter being caused by a deficiency of clotting factor IX. Patients with hemophilia face the daily threat of spontaneous bleeding episodes that can lead to serious joint damage, fatigue, and lifestyle limitations. As a result, individuals must be vigilant and often rely on regular prophylactic treatment involving intravenous injections of clotting factors to manage their condition and maintain a semblance of normalcy in their lives.
Beyond the physical impacts, hemophilia also has profound social implications. Many individuals with this condition grapple with anxiety and feelings of isolation due to the constant need to explain their restrictions to peers and the fear of bleeding episodes. Terence Blue’s experience underscores this struggle; from an early age, he learned the importance of being cautious to prevent harmful injuries. Despite the advancements in hemophilia treatment, such as synthetic clotting factors, the emotional toll remains a significant aspect of living with this disorder.
A New Era in Hemophilia Treatment: Gene Therapy Breakthrough
Gene therapy for hemophilia represents a revolutionary development in the field of medicine, particularly for patients with hemophilia B. The FDA-approved Hemgenix therapy enables a single infusion to introduce a corrected version of the gene responsible for producing clotting factor IX directly into a patient’s liver. This innovative approach has shown promising results, with many patients achieving unprecedented sustained levels of factor IX and independence from continuous factor replacement therapy. This breakthrough in gene therapy holds great potential for significantly improving the quality of life for individuals managing hemophilia.
The advantages of gene therapy extend beyond just patient outcomes; they also pose a critical challenge in the healthcare market. While the scientific community celebrates the launch of treatments like Hemgenix, concerns arise regarding cost and accessibility. The price tag of around $3.5 million raises questions about healthcare affordability and the sustainability of such therapies. Nonetheless, the positive initial results from treatment recipients have sparked hope for broader adoption and ongoing development in gene therapy for hemophilia, emphasizing the urgent need for economic models that support innovation while ensuring patient access.
Hemgenix and similar breakthroughs could potentially revolutionize how hemophilia is treated, giving patients the freedom to live with less fear of bleeding complications. Many are optimistic that gene therapies will continue to enhance treatment options and shift the paradigm of hemophilia management from lifelong treatment regimens to more sustainable, long-term solutions.
The Impact of Gene Therapy on Patient Experiences
For patients like Terence Blue, receiving gene therapy has been a life-changing experience. The prospect of shedding daily needles and the relentless anxiety that accompanies hemophilia management is intoxicating. The infusion of Hemgenix, which aims to provide a one-time fix, marks a turning point in how Blue and similar patients can approach their health. Though it’s early in the post-treatment journey, initial results indicated that Blue’s factor IX levels surged significantly, suggesting a newfound ability to heal and live without the constraints of traditional therapy.
As these advancements unfold, the potential for improved patient experiences will depend largely on how the medical community and healthcare systems respond. Proper education on the benefits and mechanics of gene therapy will ensure patients feel informed and supported. Enhanced collaboration between healthcare providers and patients can foster a better understanding of these therapies’ capabilities and limitations, paving the way for a brighter future where living with hemophilia is less about managing crises and more about experiencing life to its fullest.
Navigating the Future of Gene Therapy in Hemophilia
The discovery and implementation of gene therapy for hemophilia mark a pivotal shift in the treatment landscape. It represents a culmination of years of research and clinical trials aimed at addressing the root cause of hemophilia B rather than merely managing its symptoms. Continued advancements in genetic engineering and therapy delivery systems will likely pave the path for more tailored solutions for various forms of hemophilia and related disorders.
Furthermore, it’s crucial for the healthcare system to anticipate the growing demand for gene therapy treatments and address any barriers to access that may arise. As evidenced by the varied reactions to newer therapies, inclusive dialogue between patients, providers, and policymakers will be essential in defining how these groundbreaking innovations can be integrated into existing treatment protocols. The ultimate goal is to ensure that the promise of gene therapy translates into tangible outcomes for all individuals affected by hemophilia.
Cost Challenges and Financial Implications of Gene Therapy
While the potential benefits of gene therapy in treating hemophilia are substantial, the cost remains a daunting barrier. With Hemgenix priced at approximately $3.5 million, questions about health insurance coverage and long-term affordability loom large. Many patients and advocates emphasize the need for insurance models and government policies that can accommodate the high upfront costs without compromising patient access or care standards. Ensuring that financial hurdles do not prevent patients from receiving this transformative treatment is critical to its broader success.
Moreover, evaluating the economics of gene therapy involves considering both the lifetime costs of ongoing treatments like clotting factor infusions and the potential savings from reduced emergency care and hospitalizations. Therefore, a shift toward preventive solutions through gene therapy could yield significant budgetary savings over time, making a compelling case for their integration into standard treatment regimens.
Patient Perspectives: Testimonials on Gene Therapy
The lived experiences of patients who have undergone gene therapy offer valuable insights into its impact. For Terence Blue, the moment he received Hemgenix signified a new chapter in his life. His reflections reveal an emotional rollercoaster as he transitioned from a routine of managing constant medical interventions to the hopeful possibility of reduced medication needs. Listening to similar testimonials can foster community support among patients and inspire those considering gene therapy to learn more about their options.
Likewise, encouraging patients to share their stories can demystify the process of gene therapy, addressing any fears surrounding new medical technologies. Understanding the journey of others like Blue can bolster the courage of potential candidates, highlighting the real-life implications of innovative treatments and reassuring them about their experiences post-treatment.
The Role of Medical Institutions in Advancing Gene Therapy
Institutions such as Harvard-affiliated Brigham and Women’s Hospital play a crucial role in advancing research and application of gene therapies like Hemgenix. By being at the forefront of clinical trials, these centers contribute invaluable knowledge while providing patients access to cutting-edge treatments. The collaboration between pioneering researchers and clinical staff ensures that innovative therapies can be effectively implemented in clinical settings, promising improved outcomes for hemophilia patients.
Moreover, the responsibility of these institutions extends into educating patients and families about new treatment options. By providing comprehensive information regarding gene therapy mechanisms and potential outcomes, they empower patients to make informed decisions about their treatment journey. A strong educational framework can ease anxiety and build trust, fostering an environment where patients feel supported in navigating the new landscape of hemophilia treatment.
Social and Psychological Aspects of Living With Hemophilia
Living with hemophilia is not solely about managing physical health; it also encompasses navigating psychological and social dimensions. The pressure to conform to societal norms while managing a chronic condition can weigh heavily on patients. For many, discussions surrounding their diagnosis and the need for lifestyle modifications create barriers to social integration, often leading to feelings of isolation.
Furthermore, the emotional toll of constantly being aware of one’s limitations can introduce anxiety and stress. Proactive approaches involving mentorship and support groups can create opportunities for patients to share their experiences and coping strategies, fostering a sense of community. Through these connections, individuals with hemophilia can learn from one another, paving the way for better emotional resilience and overall well-being.
The Future of Hemophilia Treatment: Hope and Expectations
Looking ahead, the future of hemophilia treatment is laden with hope and promise. The continued evolution of gene therapy is anticipated to lead to a broader spectrum of potential therapies for various genetic disorders. With increased understanding of gene editing technologies and viral delivery mechanisms, researchers are well-equipped to tackle challenges and usher in new treatments that can offer long-lasting solutions.
Patient advocacy and awareness efforts will be vital in ensuring that advancements in treatment reach those who need them most. By galvanizing public support and fostering discussions about the needs and experiences of the hemophilia community, stakeholders can help ensure that innovative therapies are both developed and accessible. As the narrative of hemophilia management shifts, the commitment to continually improving care methods and patient support will remain at the forefront of the conversation.
Frequently Asked Questions
What is Hemgenix gene therapy for hemophilia B?
Hemgenix is a groundbreaking gene therapy for hemophilia B, developed to address the underlying genetic issue causing this condition. By using a virus to deliver a corrected copy of the clotting factor IX gene, Hemgenix enables patients to produce their own clotting factor, drastically improving their quality of life and reducing reliance on regular infusions of clotting factor.
How does gene therapy for hemophilia differ from traditional hemophilia treatment?
Traditional hemophilia treatments involve regular infusions of clotting factor IX to prevent bleeding episodes. In contrast, gene therapy for hemophilia, such as Hemgenix, aims to correct the underlying genetic defect by introducing a functional gene into the patient’s liver, potentially providing a long-lasting solution after just a single administration.
What are the benefits of using gene therapy for patients with hemophilia?
Gene therapy for hemophilia offers several significant benefits, including a potential reduction in the frequency of bleeding episodes, decreased dependency on regular infusions of clotting factor IX, and improved overall quality of life. Patients like Terence Blue have reported healing more quickly and enjoying activities without the constant worry associated with their condition.
Is gene therapy for hemophilia B a permanent solution?
While gene therapy for hemophilia B, such as Hemgenix, shows promising results in increasing clotting factor IX levels and reducing bleeding, it is important to note that it is not universally considered a ‘cure.’ Some patients may experience long-lasting effects for years, with many still not requiring factor IX infusions months after treatment.
What is the FDA’s role in approving gene therapies like Hemgenix for hemophilia?
The FDA plays a critical role in ensuring the safety and efficacy of gene therapies like Hemgenix for hemophilia before they are approved for public use. In November 2022, the FDA granted approval to Hemgenix based on its demonstrated ability to improve clotting factor IX levels in clinical trials, bringing hope to patients with hemophilia B.
What challenges are associated with the market for gene therapy in hemophilia treatment?
Despite the promise of gene therapy for hemophilia, challenges include high treatment costs, such as Hemgenix being priced at $3.5 million, and varying levels of patient acceptance. These market pressures could affect the accessibility and long-term viability of gene therapies, despite their potential benefits in treating hemophilia.
What are the potential side effects of Hemgenix gene therapy for hemophilia B?
Patients undergoing Hemgenix gene therapy may experience mild to moderate side effects, including elevated liver enzymes, which is managed through monitoring and steroid treatment as needed. Most patients report few adverse effects during and after the administration of the therapy.
How has gene therapy for hemophilia changed patients’ lives?
Gene therapy for hemophilia, particularly with treatments like Hemgenix, has significantly transformed patients’ lives by reducing the need for regular clotting factor infusions, allowing them to engage in activities without the fear of bleeding complications. Patients have reported feeling healthier and healing faster, leading to enhanced psychological well-being.
| Key Point | Details |
|---|---|
| Terence Blue’s Experience | Terence Blue became the first patient in New England to receive Hemgenix, a gene therapy for hemophilia B. |
| Gene Therapy Approval | Hemgenix was approved by the FDA in November 2022 and aims to offer long-term relief from hemophilia symptoms. |
| Market Challenges | High costs and limited patient acceptance are significant challenges facing gene therapies, including Hemgenix. |
| Mechanism of Action | The therapy involves a virus that introduces a corrected gene into liver cells to produce clotting factor IX. |
| Impact on Quality of Life | Patients like Terence aim for a life free of daily injections and the anxiety of bleeding episodes. |
| Future of Gene Therapy | Despite market pressures, optimism exists for the continued development of effective gene therapies for hemophilia and beyond. |
Summary
Gene therapy for hemophilia represents a groundbreaking advancement in treatment options for this chronic condition. Recent developments, especially the use of Hemgenix, highlight the potential to significantly improve the quality of life for patients like Terence Blue, who have lived with the burdens of daily injections and constant worry about bleeding. The optimism surrounding gene therapy is palpable as it not only provides a chance for long-term solutions but also marks a significant step toward the potential for a cure in future.