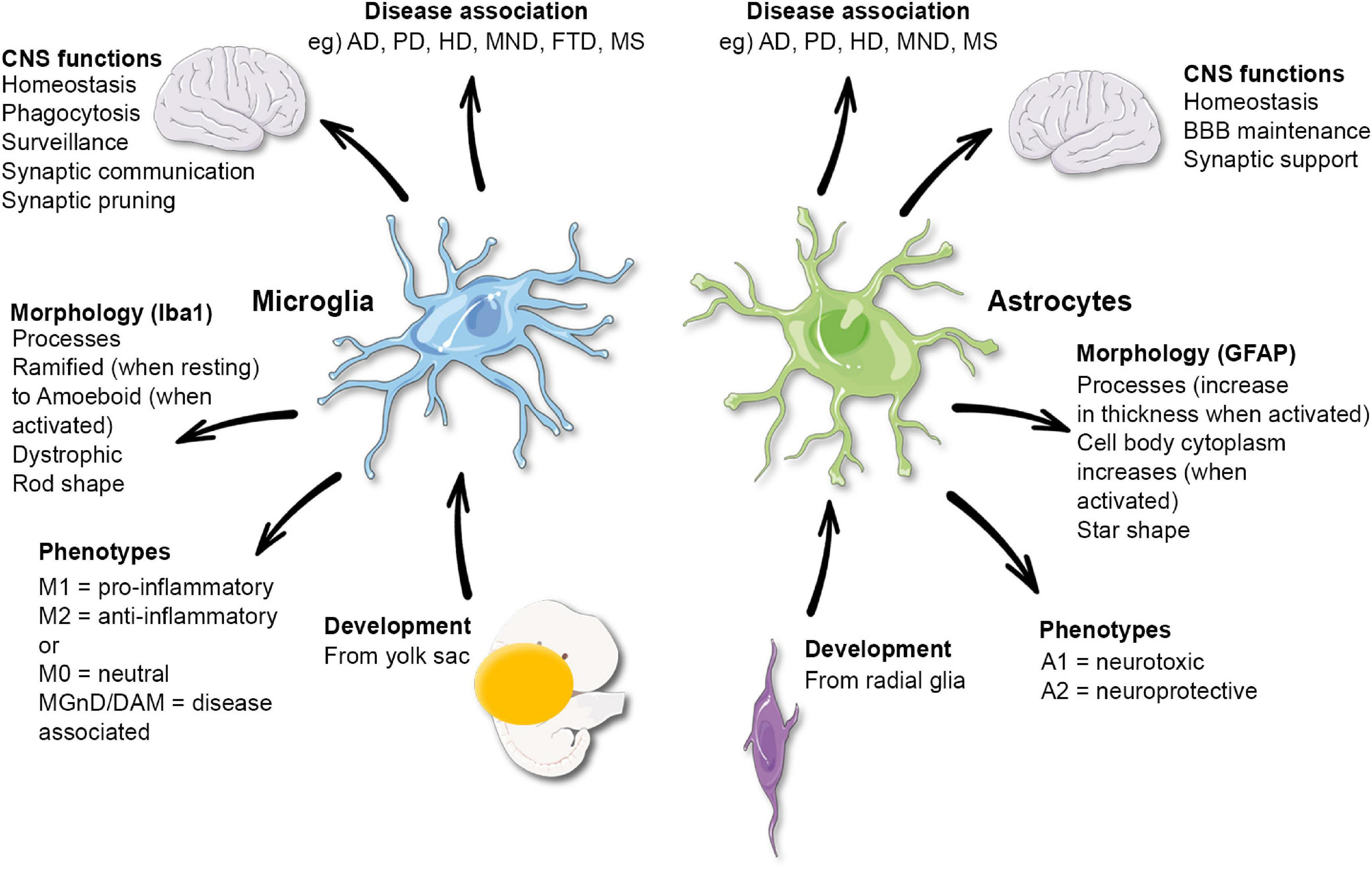
Microglial research is revolutionizing our understanding of the brain’s immune system, particularly in the context of Alzheimer’s disease and other neurodegenerative diseases. These remarkable cells monitor brain health by removing dead neurons and regulating synaptic pruning, a vital process for maintaining optimal neural function. However, recent findings by leading neuroscientist Beth Stevens reveal that when microglial activity goes awry, it can exacerbate conditions like Alzheimer’s disease and Huntington’s disease. Located at the forefront of this exciting field, the Stevens Lab at Boston Children’s Hospital demonstrates how these immune cells are not only defenders of brain health but also critical players in the pathology of these devastating disorders. The implications of microglial dysfunction underscore the necessity for continued research, which promises new therapeutic avenues for the millions affected by these conditions.
Investigations into glial cells have opened up a new frontier in neuroscience, particularly regarding their role in neurodegenerative disorders such as Alzheimer’s disease. Known as microglia, these brain-resident immune cells are essential for maintaining neural integrity and functioning, engaging in tasks such as the meticulous process of synaptic pruning. Errors in this pruning mechanism can lead to neurodegeneration, highlighting the intricate relationship between the brain’s immune response and neuronal health. Prominent researchers like Beth Stevens are at the helm of exploring these dynamics, uncovering how malfunctioning microglial processes can contribute to the pathology of various debilitating diseases. As we gain deeper insights, microglial investigations are poised to unveil promising strategies for diagnosing and treating conditions that currently impact millions worldwide.
Understanding Microglial Cells: The Brain’s Immune System
Microglial cells are crucial components of the brain’s immune system, primarily responsible for monitoring and maintaining the health of neuronal environments. They act as sentinels, constantly surveying the brain for signs of damage, disease, or infection. When they detect abnormalities, microglia spring into action—removing dead cells, repairing neural connections, and pruning synapses that may no longer be effective. This dynamic response is essential for brain homeostasis and normal cognitive functions, making it imperative to understand how microglial activity impacts neurodegenerative diseases such as Alzheimer’s.
In recent studies led by neuroscientist Beth Stevens, the transformative role of microglial cells has been highlighted in the context of Alzheimer’s disease and other neurodegenerative disorders. Stevens’s findings reveal that while microglia are designed to protect the brain, their overactive response can lead to synaptic pruning that may exacerbate conditions like Alzheimer’s. By unraveling the mechanisms of microglial function, researchers can explore new therapeutic strategies aimed at regulating these immune cells, addressing root causes rather than merely managing symptoms of neurodegenerative diseases.
The Implications of Synaptic Pruning on Neurodegenerative Diseases
Synaptic pruning is a natural process that allows the brain to refine its neural circuits, optimizing communication between neurons. However, dysregulation of this process can have dire consequences for brain health, particularly in the context of Alzheimer’s disease. Researchers have found that improper synaptic pruning by microglial cells can result in excessive loss of synapses, ultimately leading to cognitive decline. This disturbing link highlights the importance of investigating how microglial behavior can be influenced to prevent synaptic loss and protect the brain from neurodegenerative diseases.
Beth Stevens’s groundbreaking research sheds light on the delicate balance that microglia maintain during synaptic pruning. By delineating the pathways that govern this process, her work paves the way for developing precise interventions. Understanding the triggers of misguided pruning can inform new therapeutic approaches aimed at restoring normal synaptic architecture in patients with Alzheimer’s. Thus, the interplay of microglial function and synaptic health is essential not only in revealing disease mechanisms but also in enhancing strategies for treatment of neurodegenerative disorders.
Beth Stevens: A Pioneer in Microglial Research
Beth Stevens has emerged as a leading figure in neuroscience, particularly through her investigation into microglial cells and their implications in diseases like Alzheimer’s. Her innovative research has transformed our comprehension of how the brain’s immune system operates, underscoring the significance of microglial regulation in maintaining cognitive function. Awarded the MacArthur Fellowship in recognition of her groundbreaking work, Stevens illustrates how curiosity-driven research can unlock vital insights into neurodegenerative diseases that affect millions.
Her lab’s contributions extend beyond understanding the pathology of Alzheimer’s. By uncovering the complexities of synaptic pruning mediated by microglia, Stevens has laid the groundwork for developing new biomarkers and therapeutic strategies. Through her relentless pursuit of knowledge, she inspires future generations of scientists to explore the unexplored realms of neurobiology, highlighting the profound impact that foundational research can have on clinical outcomes for conditions such as Alzheimer’s and beyond.
The Role of Federal Funding in Advancing Neuroscience Research
Federal funding plays a pivotal role in the advancement of neuroscience research, particularly in fields addressing pressing health issues such as Alzheimer’s disease. As exemplified in Beth Stevens’s journey, grants from the National Institutes of Health (NIH) provided crucial resources that enabled her to explore the intricate functions of microglial cells. Such funding supports the basic science necessary for breakthroughs in understanding the brain’s immune system, spurring innovation and launching projects that may lead to groundbreaking treatments for neurodegenerative diseases.
The sustained investment from federal agencies not only facilitates individual research projects but also fosters a collaborative environment where scientists can share their findings and insights. Through these collaborative efforts, researchers are able to build upon one another’s work, creating a solid foundation for future discoveries. As key funding sources continue to support neuroscience initiatives, hope remains alive for developing effective strategies against Alzheimer’s and similar disorders, ultimately transforming care for the millions affected.
Exploring Biomarkers for Alzheimer’s Disease Detection
The identification of potential biomarkers for Alzheimer’s disease represents a crucial frontier in neuroscience, enabling earlier diagnosis and more targeted therapies. Research in the Stevens Lab has emphasized the role of microglial activity as a promising area for biomarker discovery. By monitoring how microglia respond to synaptic changes and pathological events in the brain, scientists are hopeful that they can develop reliable indicators for Alzheimer’s, improving the chances of timely intervention and effective management of the disease.
Developing biomarkers not only aids in diagnosis but also enhances our understanding of disease progression. Through advanced imaging techniques and molecular profiling, researchers can track changes in microglial function over time, potentially correlating these changes with clinical outcomes. This multidisciplinary approach highlights the integration of biological research and medical applications, bridging the gap between bench and bedside in the fight against Alzheimer’s disease.
Synaptic Connections and Their Impact on Cognitive Function
Synaptic connections serve as the foundation for communication within the brain, directly influencing cognitive functions like memory and learning. The health and stability of these connections are critical, and any dysregulation can lead to cognitive decline commonly seen in Alzheimer’s disease. As microglial cells prune synapses based on neuronal activity, understanding how they achieve this balance is essential for promoting healthy cognitive aging and preventing neurodegenerative disease.
Beth Stevens’s research underscores the complex relationship between microglia and synaptic health, revealing that while pruning is necessary for healthy brain function, excessive or misdirected pruning can contribute to conditions like Alzheimer’s. By exploring the mechanisms regulating synaptic connectivity, researchers can aim to develop interventions that enhance or protect synapses, fostering resilience against cognitive impairments associated with neurodegenerative diseases.
Neurodegenerative Diseases: The Need for Innovative Therapies
As the population ages, the prevalence of neurodegenerative diseases such as Alzheimer’s continues to rise, creating an urgent need for innovative therapies. Traditional treatment approaches have struggled to manage symptoms effectively, emphasizing the importance of research that targets underlying mechanisms. Insights from microglial research, particularly in the context of synaptic pruning, may hold the key to developing new therapeutic strategies that not only treat symptoms but also prevent or slow disease progression.
Understanding the role of microglia in health and disease provides a pathway toward creating disease-modifying therapies. By targeting the inappropriate activity of microglial cells, researchers can design interventions that restore balance and promote neuronal resilience. The ongoing investigation into the immune functions of the brain presents exciting opportunities for breakthroughs in neurodegenerative disease therapy, establishing a new frontier in neuroscience and healthcare.
The Future of Neuroscience: Advances in Microglial Research
The future of neuroscience lies in the continued exploration of microglial cells and their role in brain health and disease. As researchers like Beth Stevens delve deeper into the properties of these critical immune cells, their findings are reshaping our understanding of neurological impairments and opening up new avenues for intervention. Innovations in imaging and molecular biology will allow for more detailed studies of microglial behavior in living brains, facilitating the identification of potential therapeutic targets.
Furthermore, as interdisciplinary collaborations grow, there is potential for leveraging diverse scientific expertise to tackle complex problems associated with neurodegenerative disorders. The integration of findings from immunology, genetics, and neuroscience promises to accelerate advances in understanding Alzheimer’s disease, ultimately leading to transformative impacts on patient care and treatment strategies. With dedicated research and support, the fight against neurodegenerative diseases is poised for significant breakthroughs.
Frequently Asked Questions
What role do microglial cells play in Alzheimer’s disease research?
Microglial cells are crucial in Alzheimer’s disease research as they act as the brain’s immune system. They are responsible for monitoring brain health, clearing away dead or damaged neurons, and participating in synaptic pruning. Disruptions in these processes can contribute to neurodegenerative diseases like Alzheimer’s.
How does synaptic pruning by microglia affect neurodegenerative diseases?
Synaptic pruning by microglia is essential for normal brain development but can become misguided in neurodegenerative diseases. In conditions such as Alzheimer’s disease, improper synaptic pruning can lead to the loss of important neuronal connections and exacerbate disease progression.
Who is Beth Stevens and what is her contribution to microglial research?
Beth Stevens is a prominent neuroscientist whose research has significantly advanced the understanding of microglial cells and their role in neurodegenerative diseases. Her work has highlighted how microglia’s function in synaptic pruning can affect the development of conditions like Alzheimer’s and laid the groundwork for potential biomarkers and treatments.
Why is microglial research important for understanding neurodegenerative diseases?
Microglial research is vital for understanding neurodegenerative diseases as it provides insights into how the brain’s immune response influences neuronal health. Understanding microglial functions can lead to new therapies that target the underlying mechanisms of diseases such as Alzheimer’s, potentially improving outcomes for millions.
What potential therapies could arise from microglial research in Alzheimer’s disease?
Microglial research holds the potential to develop novel therapies for Alzheimer’s disease by identifying biomarkers and signaling pathways involved in synaptic pruning and immune response. These insights could guide the creation of targeted treatments aimed at correcting harmful microglial activity and preserving cognitive function.
How do microglia interact with the brain’s immune system and influence Alzheimer’s disease?
Microglia are an integral part of the brain’s immune system, providing support through the clearance of debris and regulation of synaptic pruning. In Alzheimer’s disease, dysfunctional microglial responses can worsen neuroinflammation and contribute to synaptic loss, highlighting their dual role in protecting and potentially harming neuronal circuits.
What foundational research led to developments in microglial research for neurodegenerative diseases?
Foundational research in microglial biology, largely supported by federal funding, has been critical for understanding their role in synaptic pruning and immune responses. This early work allowed researchers like Beth Stevens to explore the implications for neurodegenerative diseases such as Alzheimer’s, paving the way for future advancements in diagnosis and treatment.
Can improper synaptic pruning by microglia lead to diseases beyond Alzheimer’s?
Yes, improper synaptic pruning by microglia can impact other neurodegenerative diseases beyond Alzheimer’s, such as Huntington’s disease. Understanding these processes can help identify common pathways in various conditions and inform new therapeutic strategies.
| Key Points |
|---|
| Microglial cells act as the brain’s immune system, monitoring for illness and injury. |
| They clear away dead or damaged cells and trim synapses for efficient neuron communication. |
| Improper pruning by microglia can contribute to various neurodegenerative diseases, including Alzheimer’s. |
| Research from the Stevens Lab is leading to potential new biomarkers and medications for Alzheimer’s care. |
| Stevens’ foundational research has been supported significantly by federal funding, primarily from NIH. |
| The work showcases the importance of basic science in understanding complex diseases and developing treatments. |
| Stevens highlights the impact of curiosity-driven research on advancements in microglial understanding. |
Summary
Microglial research plays a crucial role in advancing our understanding of brain health and disease. Beth Stevens’ work reveals the dual nature of microglial cells as both protectors and potential contributors to neurodegenerative diseases like Alzheimer’s. The importance of curiosity-driven research and federal support is pivotal in making strides toward finding effective treatments and improving the lives of millions affected by such conditions.