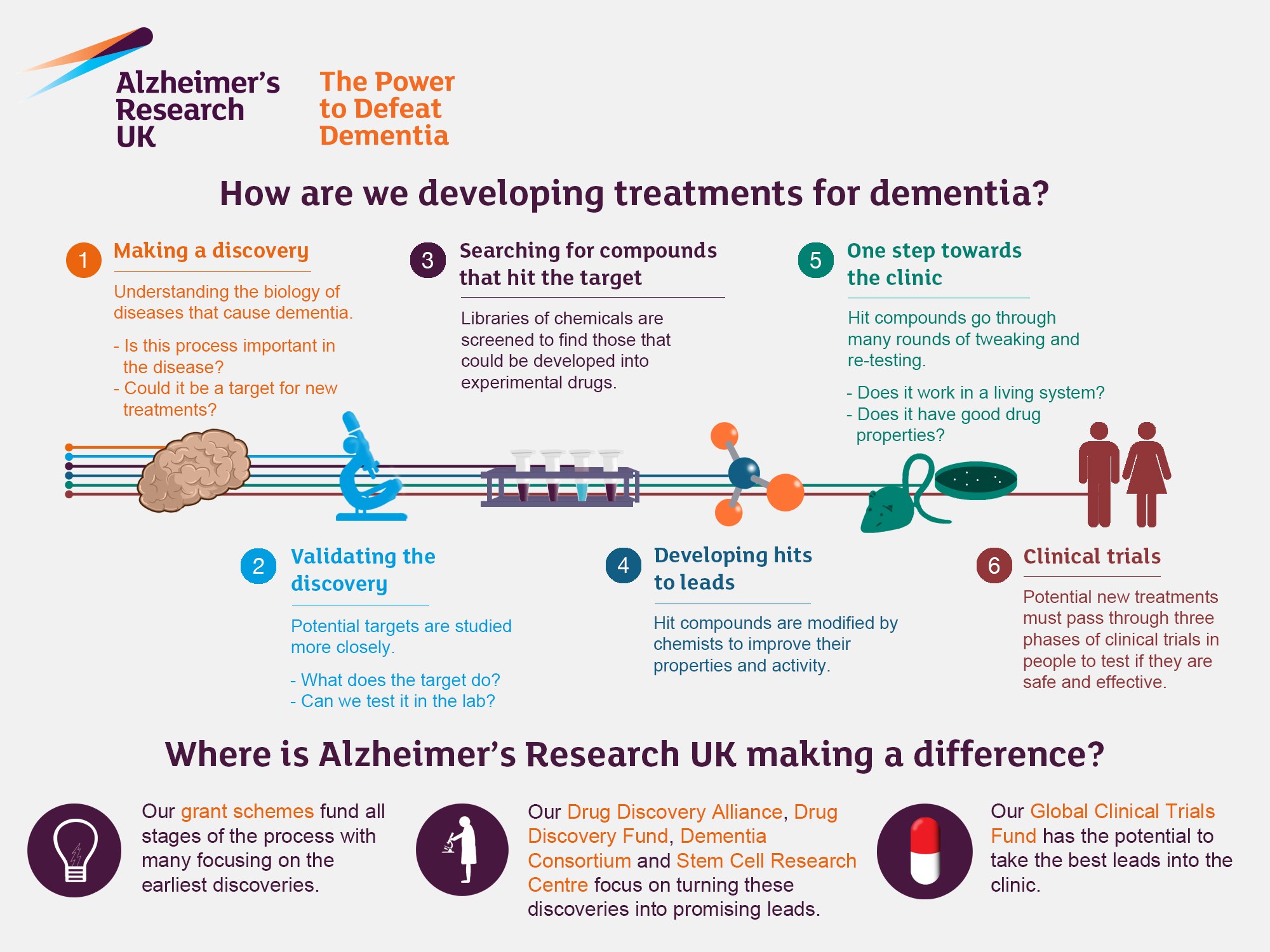
Alzheimer’s research is at the forefront of the battle against one of society’s most pressing health crises. Pioneering neuroscientist Beth Stevens is reshaping our understanding of microglial cells, which serve as the brain’s immune system and play a crucial role in combating neurodegenerative diseases. These cells not only identify and eliminate damaged cells but also prune synapses essential for communication between neurons. However, Stevens’ groundbreaking studies revealed that improper pruning may contribute to diseases such as Alzheimer’s and Huntington’s. With approximately 7 million Americans affected by Alzheimer’s, her innovative findings pave the way for new Alzheimer’s treatment strategies and potential early biomarkers that could drastically alter the course of this catastrophic illness.
The exploration of Alzheimer’s disease encompasses a broad range of studies aimed at understanding its underlying mechanisms and potential therapies. By investigating the role of brain immune cells, researchers like Beth Stevens have shed light on the processes that can lead to cognitive decline and memory loss associated with neurodegenerative conditions. Her work focuses on how these immune cells, critical to maintaining brain health, can malfunction and exacerbate this devastating disorder. This comprehensive approach to studying Alzheimer’s not only aims to develop effective therapies but also emphasizes the importance of early detection in managing the disease. As new insights emerge from such research, we move closer to innovative interventions that could transform outcomes for millions affected by this condition.
Beth Stevens: A Leader in Alzheimer’s Research
Beth Stevens has emerged as a pioneering figure in the field of Alzheimer’s research, focusing on the role of microglial cells as essential components of the brain’s immune system. Her groundbreaking studies have challenged traditional perceptions of these cells, revealing their significant involvement not only in normal brain development but also in the pathology of neurodegenerative diseases. With more than 7 million Americans currently affected by Alzheimer’s, Stevens’ work is crucial in understanding how malfunctioning microglial cells contribute to the progression of this debilitating illness.
At Boston Children’s Hospital and the Broad Institute, Stevens leads a dedicated team that is investigating the complex interactions between microglia and neurons. Through her research, it has been established that improper pruning of synapses by microglia can lead to the early onset of Alzheimer’s disease, significantly impacting cognitive function. Understanding these cellular processes is vital for developing effective Alzheimer’s treatments that may help mitigate the symptoms and progression of the disease for millions in the coming decades.
The Role of Microglial Cells in Neurodegenerative Diseases
Microglial cells, often referred to as the brain’s immune defenders, play a pivotal role in maintaining the health of neural circuits. They are responsible for the surveillance of the brain environment, assisting in the removal of debris and dead neurons through a process known as phagocytosis. However, when microglial function is compromised, as seen in various neurodegenerative diseases including Alzheimer’s and Huntington’s disease, the consequences can be dire. Stevens’ research highlights the need to harness the potential of microglia for therapeutic interventions.
Stevens’ innovative approaches have revealed how dysfunctional microglial activity can lead to synaptic loss, a hallmark of Alzheimer’s disease. She emphasizes that understanding the mechanisms behind this faulty pruning is crucial for establishing biomarkers that could enable earlier diagnosis and more effective treatment options. Stimulating proper microglial function represents a promising avenue in the fight against neurodegenerative diseases, potentially altering the course of these devastating conditions.
Advancements in Alzheimer’s Treatment through Basic Science
Stevens emphasizes the importance of basic research in the field of Alzheimer’s treatment, arguing that discoveries can take years or even decades to translate into practical applications. Her assertion is that each step down the path of basic science can illuminate new potential interventions and lead to advancements in clinical treatments. The breakthroughs in understanding microglial cells and their role in synaptic health reflect how foundational research can have far-reaching implications for diseases affecting millions.
The ongoing pursuit of knowledge in the neuroscience field has the potential to develop novel therapies that target the underlying mechanisms of Alzheimer’s disease. By focusing on the interactions between microglia and neurons, researchers like Stevens are building a bridge between basic science and clinical outcomes, bringing hope to patients and families affected by Alzheimer’s. These efforts signify a transformation in the approach to treating neurodegenerative diseases, moving toward a more proactive strategy that anticipates and mitigates the effects of such illnesses.
Funding in Neuroscience Research: A Critical Component
Stevens credits substantial federal funding as instrumental in facilitating advancements in neuroscience research, particularly regarding Alzheimer’s. The support from agencies like the National Institutes of Health has enabled her lab to explore complex biological questions that require extensive resources and time to address. Without this foundational backing, many of the critical experiments that have led to today’s understanding of microglial function and its implications for Alzheimer’s may not have been possible.
Moreover, ongoing government and private funding remain vital to sustaining momentum in Alzheimer’s research. As the aging population grows, the urgency for innovative treatments increases dramatically. Investment in this area not only propels essential discoveries but also reflects a societal commitment to addressing one of the most pressing public health challenges of our time. The financing of dedicated research efforts will likely yield new insights into Alzheimer’s treatment, offering hope for improved quality of life for those affected.
Exploring Synaptic Pruning and Alzheimer’s Disease
One of the most intriguing aspects of Beth Stevens’ research lies in exploring the concept of synaptic pruning and its direct impact on Alzheimer’s disease. Microglial cells play a crucial role in determining which synapses should be retained and which should be eliminated based on neuronal activity. However, when this process becomes dysregulated, as seen in Alzheimer’s, the consequences can be catastrophic for neural communication and overall brain function.
Understanding synaptic pruning provides critical insights into the pathological processes of Alzheimer’s disease. Stevens’ work suggests that enhancing proper pruning mechanisms could be a viable strategy for Alzheimer’s treatment, potentially leading to the preservation of cognitive functions. As researchers delve deeper into how microglial cells modulate synaptic health, they are uncovering pathways that may lead to innovative therapeutic strategies.
Malfunctioning Microglia: Impacts on Cognitive Health
The malfunctioning of microglial cells is increasingly recognized as a significant contributing factor in the decline of cognitive health, particularly in individuals suffering from Alzheimer’s disease. Instead of supporting cognitive function through active synapse maintenance, compromised microglia may exacerbate inflammation, leading to further deterioration of neuronal networks. This relationship underscores the dual role that microglial cells play, marking them as both protectors and potential contributors to neurodegeneration when misregulated.
Research led by Beth Stevens sheds light on how interventions targeting microglial function could halt or even reverse these damaging processes. By fostering an environment where microglial cells can operate efficiently, there is hope to protect cognitive health in patients at risk for Alzheimer’s. These findings represent an important step towards understanding the biological underpinnings of cognitive decline and developing interventions that could transform patient care.
Novel Biomarkers in Alzheimer’s Diagnosis
A crucial aspect of Beth Stevens’ research is the identification of novel biomarkers for Alzheimer’s disease through the study of microglial function. By understanding how these immune cells interact with neurons and contribute to disease pathology, researchers can begin to pinpoint specific biological markers that herald the onset of Alzheimer’s. Early detection is key to improving patient outcomes, and these biomarkers could allow for interventions at a stage when they are most effective.
The drive to develop reliable biomarkers reflects a broader shift in Alzheimer’s research, emphasizing the importance of preventative measures alongside treatment. Stevens’ work has laid the groundwork for future studies aimed at creating a biomarker profile that accurately reflects the health of an individual’s brain immune system. Establishing such markers could not only facilitate earlier diagnoses but also aid in the monitoring of disease progression and treatment response in Alzheimer’s patients.
The Future of Alzheimer’s Research: Collaborative Approaches
The future of Alzheimer’s research will rely heavily on collaborative efforts that bridge various disciplines, from basic neuroscience to clinical applications. Stevens advocates for a multidisciplinary approach to unveil the complexities of Alzheimer’s pathology. Collaboration among researchers, clinicians, and data scientists will drive innovation, blending insights across fields to tackle this multifaceted disease.
Such collaborative frameworks are essential for accelerating discoveries in Alzheimer’s treatment and understanding. By combining expertise, resources, and perspectives, the scientific community can collectively advance the field, ensuring that critical findings from foundational research translate into practical solutions for those living with Alzheimer’s. The integration of various scientific disciplines represents a promising paradigm shift toward finding effective interventions and one day, a potential cure for neurodegenerative diseases.
Community Impact of Alzheimer’s Research
The impact of Beth Stevens’ Alzheimer’s research extends beyond the laboratory, affecting communities and families grappling with the disease. By addressing the underlying mechanisms of Alzheimer’s through her studies on microglial cells, Stevens’ findings empower families to understand the biological aspects of the disease. This knowledge is crucial as it provides hope and informs discussions about care, treatment options, and future prospects for loved ones affected by Alzheimer’s.
Moreover, the societal implications of Stevens’ work resonate with the growing awareness of Alzheimer’s as a public health crisis. Efforts to translate research into community resources, such as educational programs and support networks, are vital in managing the emotional and financial burdens that accompany the disease. As research continues to evolve, the integration of scientific findings into community outreach will enhance the capacity to care for individuals living with Alzheimer’s, promoting a more informed and supportive society.
Frequently Asked Questions
What role do microglial cells play in Alzheimer’s research?
In Alzheimer’s research, microglial cells are recognized as the brain’s immune system, patrolling for illness or injury. They help remove dead cells and prune synapses, which is essential for healthy brain function. Beth Stevens’ research has found that faulty pruning by microglia can contribute to the progression of Alzheimer’s disease, highlighting their crucial role in neurodegenerative diseases.
How has Beth Stevens contributed to the understanding of Alzheimer’s treatment?
Beth Stevens has significantly advanced the understanding of Alzheimer’s treatment through her research on microglial cells. Her work has revealed how these cells can improperly prune synapses, leading to neurodegenerative diseases, including Alzheimer’s. By understanding these mechanisms, her research aims to identify new treatment options and biomarkers for earlier detection of Alzheimer’s.
Why are microglial cells essential in the study of neurodegenerative diseases like Alzheimer’s?
Microglial cells are essential in neurodegenerative disease studies because they function as the brain’s immune defense, involved in removing cellular debris and regulating synaptic connections. In Alzheimer’s, improper functioning of these cells has been linked to disease progression. Research by scientists like Beth Stevens aims to explore and leverage these cellular behaviors for future Alzheimer’s treatments.
What are the implications of microglial cell research for Alzheimer’s disease prevention?
Research on microglial cells has significant implications for Alzheimer’s disease prevention. By understanding how these immune cells mismanage synaptic pruning and contribute to neurodegeneration, scientists like Beth Stevens are paving the way for new treatment strategies and potential ways to prevent the onset of Alzheimer’s, providing hope for millions at risk.
How does understanding the brain’s immune system aid Alzheimer’s research?
Understanding the brain’s immune system, particularly through the study of microglial cells, aids Alzheimer’s research by providing insights into the mechanisms of disease pathology. Beth Stevens has demonstrated that the immune response in the brain can influence Alzheimer’s development, which helps researchers develop targeted treatments and interventions to mitigate disease effects.
What future directions does research on microglial cells suggest for Alzheimer’s treatment?
Research on microglial cells suggests future directions for Alzheimer’s treatment that include developing therapies aimed at correcting faulty synaptic pruning and enhancing the immune response of the brain. Insights from Beth Stevens’ lab could lead to innovative strategies that not only treat symptoms but potentially alter the disease’s trajectory.
How can the work of Beth Stevens influence the future of Alzheimer’s research?
Beth Stevens’ work has the potential to greatly influence the future of Alzheimer’s research by providing foundational knowledge about microglial cell functions and their impact on synaptic health. This could lead to novel therapeutic approaches and biomarkers for early detection, ultimately improving outcomes for individuals affected by Alzheimer’s disease.
What are the current challenges in Alzheimer’s research that microglial studies aim to address?
Current challenges in Alzheimer’s research include the early detection of the disease and identifying effective treatments. Studies on microglial cells, led by researchers like Beth Stevens, aim to address these issues by clarifying how immune responses in the brain contribute to neurodegeneration, which can inform better diagnostic tools and therapeutic strategies.
| Key Point | Details |
|---|---|
| Researcher Profile | Beth Stevens is a neuroscientist at Boston Children’s Hospital and the Broad Institute of MIT and Harvard. |
| Research Focus | Stevens’ research centers on microglial cells, which are integral to the brain’s immune system. |
| Microglial Function | They remove damaged cells and prune synapses; however, faulty pruning may contribute to neurodegenerative diseases. |
| Diseases Targeted | Research links faulty microglial function to Alzheimer’s, Huntington’s, and other disorders. |
| Future Implications | Her discoveries could lead to new medications and biomarkers for early detection of diseases. |
| Impact of Aging Population | With 7 million affected, Alzheimer’s cases are projected to double by 2050. |
| Funding and Research Foundation | Stevens emphasizes the importance of federal funding, primarily from NIH, for advancing her research. |
| Basic Science Value | Fundamental research forms the basis for innovative treatments and understanding of diseases. |
Summary
Alzheimer’s research has made significant strides due to the dedicated efforts of scientists like Beth Stevens, whose exploration of microglial cells has unveiled critical insights into the brain’s immune system. Her groundbreaking work demonstrates how understanding the processes of cell function can influence treatments for Alzheimer’s disease and improve the quality of life for millions. As we delve deeper into the complexities of the disease, it becomes increasingly evident that foundational research is essential for developing innovative strategies to combat Alzheimer’s and safeguard future generations.